Spectrum Analysis Chart JLab Manufacturer,Supplier and Exporter in India

Product Code : JL-PI-832
Jlab Export is a leading Spectrum Analysis Chart JLab Manufacturer,and suppliers in India, Spectrum Analysis Chart JLab Manufacturer,and suppliers in South Africa, Spectrum Analysis Chart JLab Algeria (Algiers), Spectrum Analysis Chart JLab Angola (Luanda), Spectrum Analysis Chart JLab Argentina (Buenos Aires), Spectrum Analysis Chart JLab Armenia (Yerevan), Spectrum Analysis Chart JLab Australia (Canberra), Spectrum Analysis Chart JLab Austria (Vienna), Bahrain (Manama), Bangladesh (Dhaka), Bhutan (Thimphu), Bolivia (Sucre), Botswana (Gaborone), Brazil (Brasília), Brunei (Bandar Seri Begawan), Montenegro (Podgorica), Morocco (Rabat), Mozambique (Maputo), Myanmar (Naypyidaw), Namibia (Windhoek), Nepal (Kathmandu), New Zealand (Wellington), Nigeria (Abuja), Oman (Muscat), Palestine (Ramallah), Panama (Panama City), Papua New Guinea (Port Moresby), Paraguay (Asunción), Peru (Lima), Philippines (Manila)¸ Portugal (Lisbon), Qatar (Doha), Rwanda (Kigali), Saudi Arabia (Riyadh), Senegal (Dakar), Serbia (Belgrade), Sierra Leone (Freetown), Slovakia (Bratislava), South Africa (Cape Town) (Pretoria) (Bloemfontein), South Sudan (Juba), Spain (Madrid), Sri Lanka (Sri Jayawardenepura Kotte) (Colombo), Sudan (Khartoum), Syria (Damascus), Tanzania (Dodoma), Thailand (Bangkok), Togo (Lomé), Tonga (Nuku'alofa), Trinidad and Tobago (Port of Spain), Tunisia (Tunis), Turkey (Ankara), Turkmenistan (Ashgabat), Uganda (Kampala), United Arab Emirates (Abu Dhabi), United Kingdom (London), United States (Washington, D.C.).
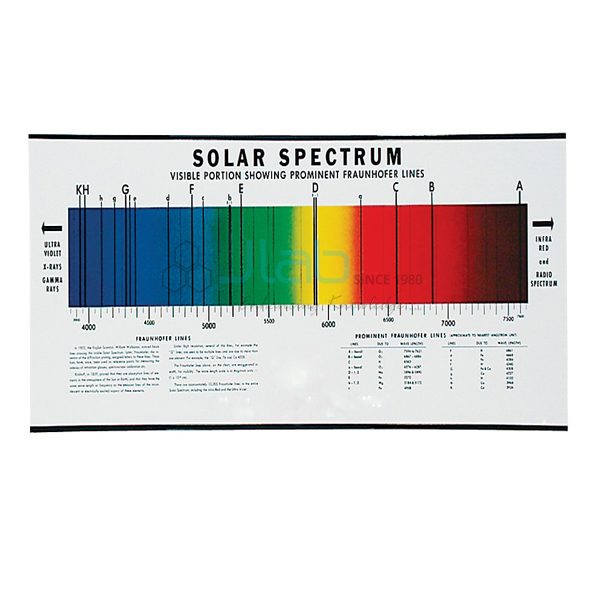
Spectrum Analysis Chart
- This chart shows the visible continuous spectrum of the sun and the emission or bright line spectra of ten relatively common elements.
- It provides an excellent beginning point to show the student the total individuality of the spectrum of each element.
- It then also presents an opportunity to discuss the general relationship between the number of lines and the number of atomic shell electrons.
- Also shown is an explanation of the “red shift” or Doppler Effect which is a change in the perceived frequency, usually of an absorption or Fraunhofer line.
- This change or shift is caused when the light source is moving at high speed toward or away from the viewer.
- Certain materials and solutions will selectively absorb one or more bands of light.
- These characteristic dark bands are very specific and readily identify the material or solution through which the light is passing.
- Absorption bands for didymium glass, chlorophyll and oxyhemoglobin are shown.
- A number of line drawings show how spectra may be produced or clarify other aspects of the importance of spectral analysis.
- This durable chart is printed on heavy paper stock and is fitted with metal binding strips at top and bottom.
- The upper strip includes a tab suitable for wall hanging.
- Dimensions
- 94 cm x 64 cm.